Some science concepts have the strange property of being both complex and simple at the same time. They may be a facet of our daily life, but, if asked, we probably wouldn’t be able to describe them well. One great example of this is acids in chemistry. From cooking to cleaning, you come in contact with acids daily; but do you actually know what they are?
Let’s discuss the question: “What is an acid?”, and take a look at three of the most common acid definitions in chemistry!
Overview of Acids
In life, acids can be extremely variable in form and function. Across all definitions, they have a few underlying characteristics, such as:
- They make food taste sour
- They have an elevated concentration of hydrogen (H+) ions
- They turn litmus paper red
- They have a pH lower than 7
- They are typically corrosive.
Acids are extremely diverse. There are many different categories of acids, which can make them hard to pin down as a major group of chemicals. There are non-organic acids (such as sulfuric and phosphoric acid) and organic acids (such as ascorbic and oxalic acid). Natural acids are often found in plants and animal systems. Ascorbic acid, also known as vitamin C, is a prime constituent in many fruits.
These are just a few of the main characteristics associated with acids. There are plenty more that you may come across, such as the reactivity of acids with various compounds.
Popular Acid Definitions
In chemistry, there are a few extremely common acid definitions that you may come across. Each describes acids as a whole, meaning they are not exclusive to specific acids. The definitions are known as Arrhenius acids, Bronsted Lowry acids, and Lewis acids.
Arrhenius Acids
One of the earliest definitions for acids came from the Swedish chemist, Svante Arrhenius. His definition is as follows:
An Arrhenius acid increases the H+ concentration within an aqueous solution.
Arrhenius acids fully dissociate in solution. Thus, they always give off H+ ions. In water, the free hydrogens combine with H20 molecules to form H3O+, also known as hydronium.
Bronsted-Lowry Acids
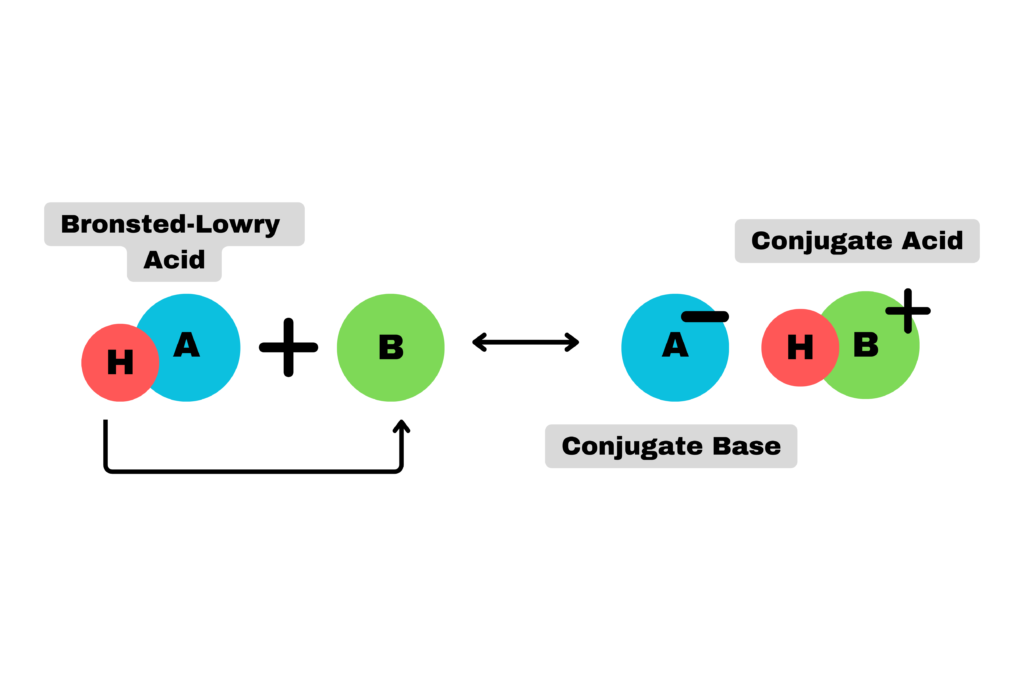
The next major definition for acids arose in 1923. It was coined by the English chemist, Thomas Lowry and Danish chemist, Johannes Bronsted. This definition goes a little more in-depth, describing acids as:
Compounds that donate a proton in solution.
In essence, Bronsted-Lowry acids describe a wider range of acid functions and depict why acids increase the proton concentration in a solution. When an acid reacts, it produces a conjugate base.
Lewis Acids
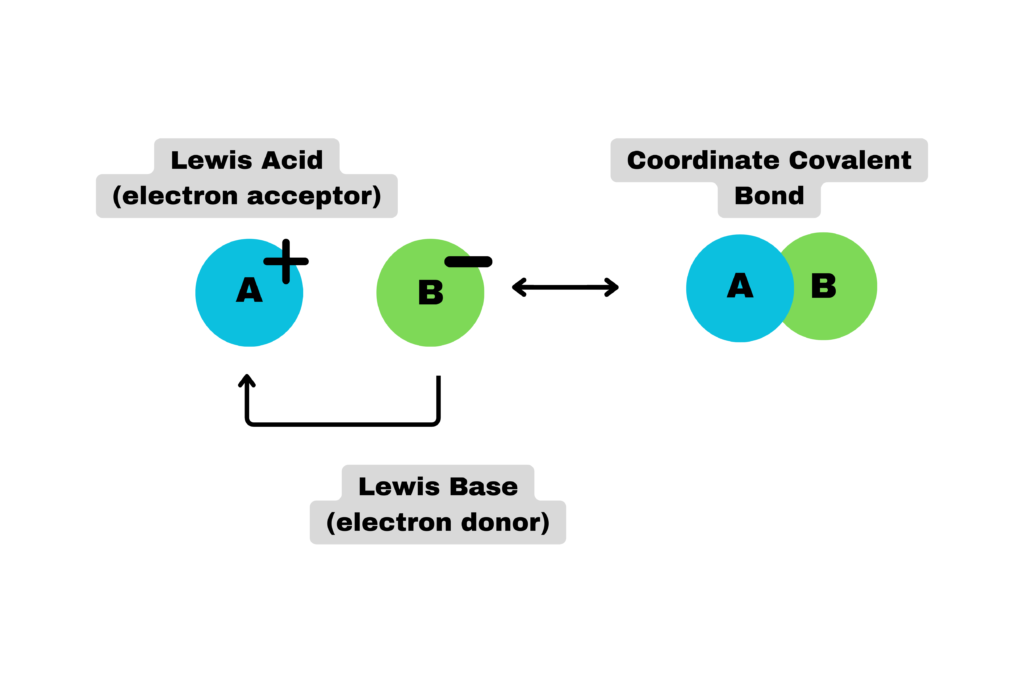
Last but not least, there are Lewis acids, which were described by G.N. Lewis in 1916. Surprisingly, his theory was not widely popularized until 1923, the same year that Bronsted-Lowry acids were proposed. Lewis acids are described as:
Substances that accept a pair of electrons, thus forming a covalent bond with the atom sharing the electrons.
As compared to the others, the definition for Lewis acids takes into account the movement of electrons and the change of both the acid and the base as a reaction occurs. As you may have guessed, Lewis acids are usually depicted with Lewis structures, as they allow for the best visualization of lone pairs and atom movement.
